- Received November 30, -0001
- Accepted November 30, -0001
- Publication May 29, 2020
- Visibility 15 Views
- Downloads 0 Downloads
- DOI 10.18231/j.ijced.2020.013
-
CrossMark
- Citation
A cross sectional study of lichen planus: It’s epidemiological, clinico-histopathogical and serological perspective
- Author Details:
-
Palakurthi Sri Sneha
-
K Seetharamanjaneyulu *
-
G Venkata Ramana
-
Satya Saya
Introduction
Lichen planus (LP) is a common papulosquamous skin disease with a prevalence of 1-2% globally and 0.1% to 1.5% in Indian studies.[1] The disease was mostly reported in middle-aged patients 30-60 years of age and is less common in extremes of age.[1] Few studies show female preponderance.[2], [3] LP most commonly involves the flexor surfaces of extremities with pruritic, purple, polygonal, planar, papules, plaques. The lesions show Wickham's striae and positive Kobners phenomenon.[4] The lesions are typically bilateral and relatively symmetrical. Strong association has been reported with HCV infection, while the association with other viral infections like EBV, CMV, Hepatitis B, HIV and Human herpes simplex virus is not that strong.[5], [6]
The association of HCV infection and lichen planus varied from the different studies. Positive association was seen in 3.1% to as high as 18.3%. More significant association was seen in Oral lichen planus.[7], [8], [9], [10], [11], [12] Few studies have found no association between HCV infection and lichenplanus.[13], [14], [15], [16], [17], [18] Though there were several reports showing a higher prevalence of HBV with LP, a possible association between HBV infection and LP remains unclear.[19], [20] Regarding association of HIV and LP, very few reports were there from India and the relation was not consistent.[21] We conducted a prospective study to assess the association of HCV, HBV, HIV infections with LP.
Materials and Methods
All patients with LP attending to our OPD, were recruited during the study period of 18 months (Jan 2017-Jun 2018). The demographic data and morphological patterns were documented. All were biopsied and histopathological features were recorded. All were tested for detection of HCV antibodies, Hepatitis B antigen and HIV antibodies by HCV-TRIDOT, HEPACARD and HIV –TRIDOT respectively.
Results
140 Lichen planus patients were enrolled. Lichen planus was seen in all age groups, however it was more common during 3rd and 4th decade. Most common age group involved was 21-30 years with mean age ± SD = 34.23 ± 12.99 years. There were 73 (52.1%)males and 67 (47.9%)females. 75.6% of the patients sought treatment with in 6 months of onset. Maximum duration of disease was 18 months and the mean duration was 4.15 ± 2.99 months. 22.9% of the patients were housemakers, 22.1% were labourers and 20.7% were students. Family history was noted in 2.9% patients. Pruritus was seen in 74% of the patients. Koebner’s phenomenon was observed in 43% of the patients. Of the total patients 5% of the patients were smokers, 4% were alcoholics, 5% were both smokers and alcoholics. Extremities were commonly involved followed by trunk. The most common clinical variants were classical LP(35%) ,followed by hypertrophic LP(31%)(Table 1). Lichen planus transforming into Keratoacanthoma was observed in one case.([Figure 1]). Oral involvement was seen in 23 (16.4%) patients. The most common site involved was buccal mucosa and reticulate pattern was seen in 79% of the oral LP. Nail involvement was seen in 3.57%. Longitudinal ridging was the commonest followed by pitting, pterygium and nail dystrophy. Cutaneous involvement alone was seen in 80% patients, Mucosal involvement alone was observed in 9.3% patients whereas both cutaneous and mucosal involvement was seen in 7.2% patients. On histopathology, hypergranulosis was most common, seen in 78% cases, followed by hyperkeratosis, basal cell degeneration and band like infiltrate in 76% cases each. Acanthosis was seen in 75% cases. and saw tooth rete ridges were seen in 57% cases. HBs Ag was positive in 4 cases, Anti HCV was positive in 3 cases, and HIV was positive in 3 cases.
| Sex | Frequency | Percent |
| Female | 67 | 47.9 |
| Male | 73 | 52.1 |
| Total | 140 | 100.0 |
| Clinical variant | Frequency | Percent |
| Actinic | 4 | 2.9 |
| Bullous | 3 | 2.1 |
| Classical | 45 | 32.1 |
| Classical and oral | 4 | 2.9 |
| Follicular | 6 | 4.3 |
| Genital | 4 | 2.9 |
| Hypertrophic | 37 | 26.4 |
| Hypertrophic and oral | 6 | 4.3 |
| Linear | 4 | 2.9 |
| LP Pigmentosus | 11 | 7.9 |
| Nail | 1 | .7 |
| Nail and oral | 4 | 2.9 |
| Oral | 9 | 6.4 |
| Palmoplantar | 2 | 1.4 |
| Total | 140 | 100.0 |
| Histopathological finding | Percentage |
| Hyperkeratosis | 76% |
| Hypergranulosis | 78% |
| Acanthosis | 75% |
| Saw toothed rete ridges | 57% |
| Basal cell degeneration | 76% |
| Band like infiltrate | 76% |
| Melanin incontinence | 70% |
| Civette bodies | 12% |
| HBsAg | Anti – HCV | HIV | |||
| Positive | Negative | Positive | Negative | Positive | Negative |
| 4 | 136 | 3 | 137 | 3 | 137 |
Discussion
Lichen Planus is a chronic inflammatory and immune mediated disease which affects skin, hair, nails, mucous membranes and appendages. Cell-mediated immunity plays a major role & humoral immunity plays a secondary role in the pathogenesis of LP. The major steps involved in the pathogenesis of LP are
LP- specific antigen recognition by CD4+Tcells and NK cells
Cytotoxic lymphocyte activation
Keratinocyte apoptosis
Lichen planus was reported commonly in 3rd and 4th decade. In our study most of the patients (55.7%) were between 21-40 yrs, similar to other studies.[22] The mean age involved in our study was 34.23 years, similar to Srivani etal in which the mean age was 37.1years.[23] LP in children was observed in 10% of the patients in our study(<18years), but it was varied from 5.43%[24] to 18%[25] in earlier studies. The low incidence reported by Shankar et al, could be due to the inclusion of children less than 12 years,[24] Whereas the other studies included up to 18 years, which could be the reason for the higher prevalence in their studies. The childhood Lichen planus in the tropics was more, may be due to early exposure to infectious agents and other environmental triggers like trauma.[25] The elder population was effected rarely. Only 2.1% of the patients were affected in our study above the age of 60 years. LP was slightly more frequently reported in males. It was seen in 52.14% of males in our study, similar to other Indian studies.[22], [26], [27], [28] The shortest duration of the disease was 15 days and the longest duration was 18months in our study. Mean duration of the disease was 4.1months and 47% of the patients were having the disease for 1-3 months, similar to the study by Shankar et al.[24] Most of the patients in our study were housewives(22.9%) followed by labourers (22.1%) and students(20.7%). Naldi etal, reported more frequently in labourers.[29] Family history was observed in 2.9% of the patients in the present study, similar to the study by Kachawa et al,[30] (2.13%). Pruritus was an important complaint in 74% of the patients in our study. Similarly Bhattacharya et al, Ireddy et al, Kachhawa et al and Abdallat et al reported pruritus in 79.3% to 82.6% of their cases.[24], [30], [31], [32] Thus pruritus is a hallmark feature of Lichen planus. In our study 15% of the patients were diabetics, and 11% of the patients were hypertensive. Of these 3.6% of these patients were both diabetic and hypertensive. Urvashi et al had found Diabetes and hypertension in 10% and 7% of their 100 cases.[28] Increased association of hypertension(22.3%) was observed in a study by Sina et al among 134 oral lichen planus patients. The most commonly involved sites in our study were lower limb (62.1%) followed by upper limb(61.4%), trunk(25.7%), and oral cavity(16.42%). Face(4.9%), neck(2.1%), scalp(3.57%), nail(3.57%), genitalia(2.9%), palms and soles(2.8%) were less involved. Urvashi et al, Salah et al, and Bhutani et al also documented that the lower limbs were the common site for LP.[28], [32], [33] Venous stasis has been implicated as a likely pathogenic mechanism for common involvement of legs. . Palms and soles (14.7%) and Nails (17.9%) were involved more in the study by Urvasi et al,[28] whereas the involvement of palms (1.4%), soles (1.4%), and nails (3.6%) was less in our study. Oral cavity was involved in 23 patients (16.5%) in our study. Reticulate, erosive pattern was observed in 79%, 21% of patients with Oral LP respectively. Shinde et al , Urvashi et al and Sreedevi et al had reported higher percentage of, 40%, 42%, 56.4%, oral involvement respectively.[27], [28], [34] The lower incidence of oral lichen planus could be attributed to the fact that the majority of the patients with oral lesions chiefly present to Dental OPD or ENT OPD.[28] The most common clinical variant observed in our study was classical papular LP followed by hypertrophic LP. In our study classical LP and hypertrophic LP were present in 35% and 30.7% respectively but in study done by Urvashi et al , classical LP and hypertrophic LP were present in 58.9 % and 28.4% respectively.[28] Palmoplantar LP and Bullous LP were the least common variants found in our study constituting 1.4% and 2.9% respectively. The palmoplantar involvement may be due to isomorphic response to trauma on palms and soles.[25]
HCV and Hepatitis B viruses have been implicated in the pathogenesis of LP. Though the exact association between Hepatitis B and LP has not been established, hepatitis B vaccines are known to trigger LP, especially after second injection.[35]
In our study, 2.9% of the patients were positive for Hepatitis B infection. Nayaf et al, and Daramola et al had reported Hepatitis B infection in 6% and 15% of their LP patients respectively.[17], [36] However studies from Uttarpradesh (India) and Nepal, had not shown any association with hepatitis B infection.[10], [13] LP could be a cytotoxic reaction to keratinocytes expressing HBsAg and not epitopes shared by hepatocytes damaged by the virus.[13]
Many studies have suggested a role of HCV infection in LP. It was more commonly reported from Japan, USA, Italy and Spain. However studies from England, France and India had not shown any significant association between HCV and LP.[37] HCV infection was reported about 1.72 -3.3% of their LP patients.[7], [10], [12], [15] HCV was more frequently associated with oral LP from Thailand, Pakistan and Saudi Arabia.[8], [9], [38] In India, studies conducted at Calicut, Kolkata, New Delhi, have failed to demonstrate a statistically significant association whereas studies conducted at Hyderabad and Bangalore have shown a significant association.[16] Pavani et al from Telangana had reported HCV infection in 12% of their Oral LP patients,[18] but a study from Puducherry did not show any association[16] The association was not consistent. In our study, 2.1% of the patients were positive for Hepatitis C virus infection.
In our study, 2.1% of the patients were positive for HIV infection. The mechanism of development of LP in HIV infected individual may be due to suppression of CD4 positive cells, differences in antigen presentation and altered immune response to antigenic stimuli.[39]
Keratoacanthoma arising from hypertrophic lichen planus was observed in one elderly male in our study with risk factors like smoking, alcohol and diabetes. ([Figure 1]) The site of involvement was lower limb and duration of the disease was 18 months. Biopsy showed pseudocarcinomatous hyperplasia with interface dermatitis in this patient. Pseudoepitheliomatous hyperplasia can be present in hypertrophic lichen planus and keratoacanthoma which can be confused with squamous cell carcinoma.[40], [41] Malignant transformation in Lichen planus is a rare phenomenon. In oral lesions, it was reported with a frequency of 1-10%, but long-standing hypertrophic or ulcerative variants of cutaneous lichen planus have 0.4% risk of malignant transformation.[40], [41] exposure, trauma, therapeutic agents and chronic inflammation may predispose to malignancy.[42]
FIGURE 1: Lichen planus transforming to keratoacanthoma
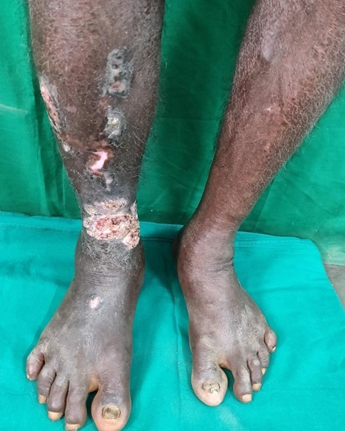
Conclusion
Lichen planus is a common papulosquamous disease commonly seen in middle aged adults usually on the extremities. Diabetes and Hypertension are associated with LP. Classical Papular LP was the commonest form, followed by Hypertrophic form. Malignant transformation can occur, so long term follow up is needed. Clinicopathological correlation has a pivotal role in providing optimal patient care. There is no relationship between LP and Hepatitis B, C and HIV virus. Hence we suggest that viral serology (HBV, HCV, HIV) for LP may not be done as a routine screening process.
Source of funding
None.
Conflict of interest
None.
References
- P Gujjar, J Zingade, S Patil, J Hallur. Recent Update on Treatment Modalities of Oral Lichen Planus-A Review. IJSS Case Rep Rev 2015. [Google Scholar]
- Michele D. Mignogna, Lorenzo Lo Muzio, Lucio Lo Russo, Stefano Fedele, Elvira Ruoppo, Eduardo Bucci, , , . Oral lichen planus: different clinical features in HCV-positive and HCV-negative patients. Int J Dermatol 2000. [Google Scholar]
- Alan S. Boyd, Kenneth H. Neldner. Lichen planus. J Am Acad Dermatol 1991. [Google Scholar]
- S Sacchidanand, Chetan Oberai, Arun C Inamdar. IADVL Textbook of Dermatology. 2015. [Google Scholar]
- Giovanni Lodi, Crispian Scully, Marco Carrozzo, Mark Griffiths, Philip B. Sugerman, Kobkan Thongprasom. Current controversies in oral lichen planus: Report of an international consensus meeting. Part 1. Viral infections and etiopathogenesis. Oral Surg, Oral Med, Oral Pathol, Oral Radiol Endodontol 2005. [Google Scholar]
- R Pellicano, F Palmas, N Leone, E Vanni, M Carrozzo, S Gandolfo. Previous tuberculosis, hepatitis C virus and lichen planus. A report of 10 cases, a causal or casual link. Panminerva Med 2000. [Google Scholar]
- Sina Gerayli, Zahra Meshkat, Alireza Pasdar, Pegah Mosannen Mozafari, Elham Banihashemi, Mohammad Amin Khajavi. The Association Between Oral Lichen Planus and Hepatitis C Virus Infection; A Report From Northeast of Iran. Jundishapur J Microbiol 2015. [Google Scholar]
- Trin Manomaivat, Surawut Pongsiriwet, Chatsri Kuansuwan, Wacharaporn Thosaporn, Kathawut Tachasuttirut, Anak Iamaroon. Association between hepatitis C infection in Thai patients with oral lichen planus: A case-control study. J Invest Clin Dent 2018. [Google Scholar]
- M Javed, M K Ahmed, M Tahir, M I Anwar. Frequency of HCV seropositivity in lichen planus. J Pak Assoc Dermatol 2017. [Google Scholar]
- E A Rajouria. Association of Lichen Planus with HCV and HBV in Nepal. Post-Graduate Med J NAMS 2011. [Google Scholar]
- A Konidena, BV Pavani. Hepatitis C virus infection in patients with oral lichen planus. Niger J Clin Prac 2011. [Google Scholar]
- Sarkis K. Strak, Khalil I. Al-Hamdi, Majid H. Alabbood. A study of lichen planus and its association with hepatitis C infection. J Taibah Univ Med Sci 2015. [Google Scholar]
- Perumal Jayavelu, Thirumal Sambandan. Prevalence of hepatitis C and hepatitis B virus infection(s) in patients with oral lichen planus. J Pharm Bioallied Sci 2012. [Google Scholar]
- Malik Adeel Anwar, Sahar Iqrar, Zain Akram, Muhammad Arslan Tayyab, Nadeem Afzal. Oral lichen planus and hepatitis C virus infection; a symbiotic relationship or a mere co-incidence?. Int J Res Med Sci 2018. [Google Scholar]
- Y Zhou, L Jiang, J Liu, X Zeng, Q M Chen. The prevalence of hepatitis C virus infection in oral lichen planus in an ethnic Chinese cohort of 232 patients. Int J Oral Sci 2010. [Google Scholar]
- Udayashankar, K Nath, D Souza M. Hepatitis Virus serology in patients with lichen planus. Int J Dermatol 2008. [Google Scholar]
- Mohammad Shahatha, Nayaf. Lichen planus and Hepatiti in Iraqi patients. Iraqi J Comm Med 2008. [Google Scholar]
- Pavani Donempudi, Harsha Bhayya, Venkateswarlu Meduri, Geetha Paramkusam, AvinashM L Tejasvi. Association of oral lichen planus with hepatitis C virus, surface antigen of hepatitis B virus, and diabetes: A clinical and biochemical study. J Indian Acad Oral Med Radiol 2016. [Google Scholar]
- K Rübsam, A Schroll, P Weisenseel, S Multhaup, T Ruzicka, J C Prinz. Lichen ruber planus und Hepatitis Virus Infektionen: kausaler Zusammenhang. JDDG: J Der Deutschen Dermatologischen Gesellschaft 2011. [Google Scholar]
- Ameet Udaykhopkar, Valia. VidyaKharkar et al Autoimmune and other associations of Lichen planus. 2013. [Google Scholar]
- Preet Mukesh Shah, Vijay Waman Dhakre. The rare occurrence of cutaneous and mucosal lichen planus in HIV infection. BMJ Case Rep 2017. [Google Scholar]
- H Bangaru, N A Karibasappa. Clinical and Histopathological Study of 50 Cases of Lichen Planus. Indian J Clin Exp Dermatol 2016. [Google Scholar]
- N Srivani, Bvn, Sravani, Shyamala, Srujana, Shravan Kumar. A study of clinical and histopathological correlation of lichen planus. Int Arch Int Med 2017. [Google Scholar]
- S G Ireddy, S G Udbalkar. Epidemiological study of lichen planus. BMR Med 2014. [Google Scholar]
- ShilpashreeP Ravikiran, AshokKumar Jaiswal, YG Anupama, NT Madan Mohan, PavanKumar Reddy. Lichen planus in children: A retrospective study in 76 patients at a tertiary care center in South India. Indian J Paediatr Dermatol 2017. [Google Scholar]
- B N Raghavendra, Jaffer Baasha, S K Et Al Clinico. Histopathological features of Lichen planus-An appraisal. Perspect Med Res 2016. [Google Scholar]
- S Deepti, P Milind, A S Rahule, W Sarang. Clinical and Histopathological Study of Lichen planus. J Cont Med A Dent 2017. [Google Scholar]
- Urvashi Tickoo, AdityaKumar Bubna, Shobana Subramanyam, Mahalakshmi Veeraraghavan, Sudha Rangarajan, Anandan Sankarasubramanian. A clinicopathologic study of lichen planus at a tertiary health care centre in south India. Pigment Int 2016. [Google Scholar]
- L Naldi, S Paolo, T Cainelli. Epidemiological evidence of association between lichen planus and two immune-related diseases. Arch Dermatol 1991. [Google Scholar]
- D Kachhawa, V Kachhawa, G Kalla, L P Gupta. A clinico-aetiological profile of 375 cases of lichen planus. Indian J Dermatol Venereol Leprol 1995. [Google Scholar]
- Madhumita Bhattacharya, Inderjeet Kaur, Bhushan Kumar. Lichen Planus: A Clinical and Epidemiological Study. J Dermatol 2000. [Google Scholar]
- S A Abdallat, T J Maaita. Epidemiological and clinical features of lichen planus in Jordanian patients. Pak J Med Sci 2007. [Google Scholar]
- L.K. Bhutani, T.R. Bedi, R.K. Pandhi, N.C. Nayak. Lichen planus pigmentosus. Dermatol 1974. [Google Scholar]
- L Sreedevi, B Reddy, S Naik, K Penchalaiah. A Study of Various Morphological types of Lichen Planus. IOSR J Dent Med Sci 2016. [Google Scholar]
- Donato Calista. Oral erosive lichen planus associated with thymoma. Int J Dermatol 2001. [Google Scholar]
- OOM Daramola, AO George, AO Ogunbiyi, JA Otegbayo. Hepatitis B virus in Nigerians with Lichen planus. West African J Med 2004. [Google Scholar]
- Raj Eady, T Burns, S Breathnach, N Cox, C Griffiths. Rook's Textbook of Dermatology. 2004. [Google Scholar]
- Asaad Tonsi, Azam Jah Samdani. Association of lichen planus with hepatitis C virus infection. Ann Saudi Med 2005. [Google Scholar]
- George E. Rippis, Brad Becker, Glynis Scott. Hypertrophic lichen planus in positive patients: A histologic immunological study. J Cutaneous Pathol 1994. [Google Scholar]
- A. Badell, J. Marcoval, I. Gallego, A. Moreno, J. Peyrí. Keratoacanthoma arising in hypertrophic lichen planus. Br J Dermatol 2000. [Google Scholar]
- B. Sigurgeirsson. Lichen planus and malignancy. An epidemiologic study of 2071 patients and a review of the literature. Arch Dermatol 1991. [Google Scholar]
- J. V. Allen. Keratoacanthomas arising in hypertrophic lichen planus. A case report. Arch Dermatol 1981. [Google Scholar]
