- Visibility 314 Views
- Downloads 50 Downloads
- Permissions
- DOI 10.18231/j.ijced.2024.077
-
CrossMark
- Citation
Role of intralesional platelet-rich plasma (PRP) therapy in the treatment of recalcitrant oral ulcers of pemphigus vulgaris
Abstract
Background: Pemphigus Vulgaris is a chronic, autoimmune vesiculobullous disorder in which 80-90% of patients develop oral lesions and in 60% of cases, oral lesions are the first sign. The platelet-rich concentrate present in autologous platelet-rich plasma (PRP) therapy has a high concentration of growth factors that promote the synthesis of collagen and extracellular matrix. The wound healing property of PRP can be used to treat recalcitrant oral ulcers of pemphigus as it accelerates the healing process, prevents the patients from side effects of steroids, and reduces the pain and discomfort of the patients.
Objective: To assess the role of intralesional Platelet Rich Plasma (PRP) therapy in the treatment of recalcitrant oral ulcers of pemphigus vulgaris.
Materials and Methods: A total of 15 histo-pathologically proven cases of Pemphigus Vulgaris under treatment, in whom the skin lesions had healed up to 90% but the oral ulcers were still persistent were included in the study. The severity of the ulcers were monitored by calculating the POLIS (Pemphigus Oral Lesion Intensity Score). Autologous intralesional PRP was administered in 3 sittings 7 days apart and the result was evaluated by change in POLIS score after each sitting. The data was analyzed using SPSS IBM version 26.0.
Observation & Results: Out of the total cases, 12 patients were female and 3 were male. Intralesional PRP was found to accelerate the healing of recalcitrant oral erosions. It was found that POLIS was reduced after every PRP session. The paired t-test was conducted to compare the mean scores of POLIS-0 and POLIS-3. The result indicates a significant difference between the pre-intervention mean score POLIS-0 (M=19, SD=3.25) and post-intervention mean score POLIS-3 (M=6.6, SD=2.97), t(14)=13.33,p<0.001.
Conclusion: Intralesional PRP has been found to improve the recalcitrant oral ulcers of pemphigus vulgaris. Hence, it can be a treatment option where oral and topical corticosteroids fail to respond or are contraindicated.
Introduction
Pemphigus Vulgaris (PV) is a chronic, recurrent, vesiculobullous disorder characterized by intraepithelial vesicles and/or bullae in the skin and mucous membranes. The mucosal dominant type of PV occurs due to auto-immune IgG antibody against Desmoglein-3 whereas both Desmoglein 1 & 3 are targeted in the mucocutaneous type of PV. 80-90% of patients with pemphigus vulgaris develop oral lesions and in 60% of cases, oral lesions are the first sign.[1] The oral ulcers are highly debilitating for the patient and difficult to treat for dermatologists as compared to skin lesions. It has been observed that oral lesions tend to persist even after the skin lesions have subsided and a prolonged course of oral/topical/intralesional corticosteroids have to be given adding considerable side effects. Topical corticosteroids cause many complications like secondary fungal and bacterial infections and these erosions also disturb the day-to-day lifestyle of patients.
Autologous platelet-rich plasma (PRP) therapy contains a platelet-rich concentrate with a large number of growth factors like platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), epithelial cell growth factor (ECGF), insulin-like growth factor (IGF), fibronectin and vascular endothelial growth factor (VEGF)[2] and has been used in the treatment of various non-healing ulcers like trophic ulcers, diabetic ulcers, neuropathic ulcers. [3] PRP promotes re-epithelialization by regulating the biological function of epidermal stem cells (ESCs). It significantly promotes the angiogenesis of wounded tissue and promotes wound contraction and stabilizes the collagen arrangement.[4] PRP also stimulates the production of collagen and extracellular matrix. The wound healing property of PRP can be used to treat recalcitrant oral ulcers of pemphigus as it accelerates the healing process, prevents the patients from side effects of steroids, and reduces the pain and discomfort of the patients.
POLIS- Pemphigus Oral Lesion Intensity Score, is a Novel Scoring System for Assessment of Severity of Oral Lesions in Pemphigus Vulgaris.[5] It evaluates 16 parameters of the disease and grades them as None (0), Mild (1), Moderate (2), Severe (3), Very severe (4). This scoring system has been used in the study to evaluate the baseline severity and monitor the response to I/L PRP therapy for recalcitrant oral ulcers in patients of PV.
|
Items |
None |
Mild |
Moderate |
Severe |
Very Severe |
|
Number of relapse (s) of the disease |
0 |
1 |
2 |
3 |
>4 |
|
Duration of oral erosions (present episode) |
<1 Week |
1 week- 3 months |
3-6 months |
6-12 months |
>12 months |
|
Number of relapse(s) of oral lesions |
0 |
1 |
2 |
3 |
>4 |
|
Persistence of oral lesions after subsidence of cutaneous lesions |
0 |
1-12 weeks |
12-24 weeks |
24-48 weeks |
>48 weeks |
|
Change in size of existing oral lesions in last one week |
Reduce by >40% |
Reduce by 30-39% |
Reduce by 20-29% |
Reduce by 10-19% |
Reduce up to 9% |
|
Number of new oral erosions in last one week |
0 |
1-3 |
4-6 |
7-9 |
>10 |
|
Difficulty in eating normal food |
None |
Mild |
Moderate |
Severe |
Very severe |
|
Difficulty in eating food according to their consistency |
None |
Mild |
Moderate |
Severe |
Very severe |
|
Difficulty in speaking |
None |
Mild |
Moderate |
Severe |
Very severe |
|
Difficulty in brushing |
None |
Mild |
Moderate |
Severe |
Very severe |
|
Difficulty in swallowing |
None |
Mild |
Moderate |
Severe |
Very severe |
|
Difficulty in mouth opening |
None |
Mild |
Moderate |
Severe |
Very severe |
|
Number of mucosae involved |
0 |
1 |
2 |
3 |
>4 |
|
Number of sites involved in the oral cavity |
0 |
1-2 |
3-5 |
6-8 |
9-11 |
|
Overall size of erosions/ulcers |
0 |
Up to 10 cm |
10-20 cm |
20-30 cm |
>30 cm |
|
Depth of the erosions |
0 |
1-10 superficial |
11-20 superficial |
21-30 superficial |
>30 superficial/ any deep erosion |
|
Total |
|
|
|
|
|
Materials and Methods
The study was conducted at a tertiary health care center in northern India for 2 years. All histopathologically proven cases of Pemphigus Vulgaris with recalcitrant oral erosions were included in the study. All the patients were treated with various modalities such as pulse dosage of corticosteroids, variable tapering doses of oral corticosteroids, injection Rituximab, immuno-suppressants like- Azathioprine, and MMF. In all such cases in which the skin lesions had almost healed (approx. 90%) but the oral lesions were still persistent and the patients were on partial remission at minimal therapy i.e. Tab Prednisolone 10 mg or equivalent and half dose of adjuvant therapy, [5], [6] were labeled as cases of Pemphigus Vulgaris with recalcitrant oral ulcers and included in the study. No topical treatment for oral ulcers was given during the study.
The baseline POLIS score was calculated and labeled as POLIS-0. All the cases included in the study were off all topical treatments for oral erosions. The cases were subjected to autologous intra-lesional PRP weekly for 3 sessions. For PRP preparation, the first centrifugation spin was done at 2400 rpm for 10 minutes and the second spin was done at 3600 rpm for 5 minutes. 5ml of freshly prepared PRP was injected intralesionally along the margins and base of the oral erosions using a tuberculin syringe. Then, the POLIS score was calculated at each session i.e. POLIS-1 at 7 days, POLIS-2 at 14 days, and POLIS-3 at 21 days. The result was evaluated by a change in POLIS score after each session. Results were analyzed using statistical software SPSS version 26.0. We performed a test of normality on our data set to guarantee the accuracy of our ensuing statistical analysis. The Shapiro-Wilk test was chosen due to the small sample (<50) of participants.
Results & Observation
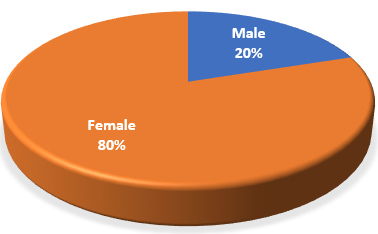
Out of the total 15 cases, 12 were female and 3 were male i.e. the gender ratio was 4:1 ([Figure 1]) ([Table 2]). The minimum and maximum age of the cases were 24 and 62 respectively ([Table 2]) with the mean age of participants being 40 ± 9.5 years.
Their disease duration ranged from 3 months to 2 years ([Table 2]).
11 patients had bilateral erosions in their buccal mucosa whereas 4 had unilateral erosions. Lips and gingiva were involved in 8 cases.
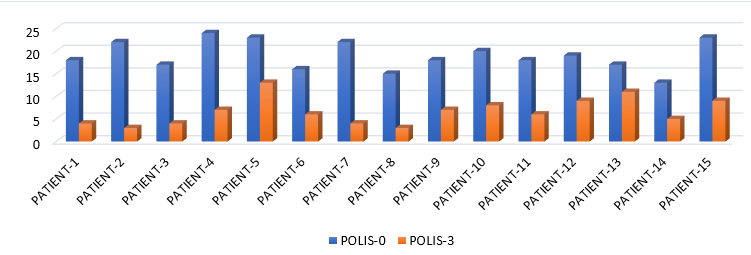
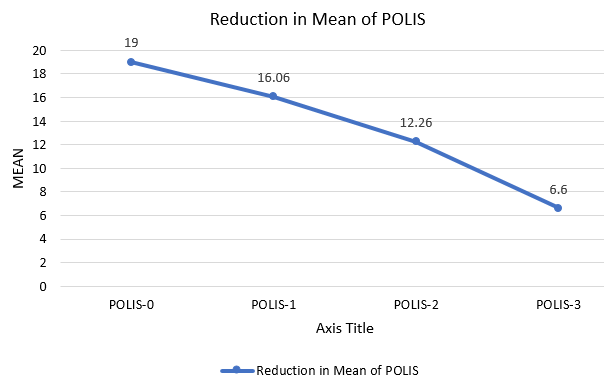
POLIS score ranges from 0 to 64.[5] The mean of POLIS-0, POLIS-1, POLIS-2, and POLIS-3 for the oral lesions were 19, 16.06, 12.26, and 6.60, respectively. It was observed that there was a gradual reduction in POLIS score after subsequent PRP sessions ([Figure 2]) and pain during brushing, eating, swallowing, and talking reduced on the Likert scale after each PRP session. There was a significant difference between the mean POLIS-0 score and POLIS-3 score in the cases, from baseline and after 3 sessions of I/L PRP therapy, (p<0.001) ([Figure 3]).
No major side effects were seen in any patients. Pain, burning sensation, and swelling during injection were complained by a few patients that subsided after some time without any treatment.
The Shapiro-Wilk test yielded a p-value larger than 0.05, meaning that the normalcy null hypothesis could not be disproved.
These findings give our analysis and interpretations a strong basis. Our data's normal distribution improves the generalizability of our conclusions.
“The t-test was conducted to compare test scores between the POLIS-0 and the POLIS-3. There was a significant difference in test scores between the baseline POLIS-0 (M = 19.0, SD = 3.25) and the POLIS-3 (M = 6.60, SD = 2.97); t(13.33) = 14, p = <0.0001.” also showing the test is significant.
|
S.No. |
Age |
Gender |
Disease Duration |
Duration of oral lesions |
Treatment received |
POLIS-0 |
POLIS-1 |
POLIS-2 |
POLIS-3 |
|
1. |
24 |
F |
8 months |
8 months |
Pulse MP RTX AZT |
18 |
16 |
10 |
04 |
|
2. |
50 |
F |
3 months |
4 months |
Pulse Dexa Pred. AZT |
22 |
18 |
09 |
03 |
|
3. |
37 |
M |
14 months |
5 months |
Pulse MP Pred. RTX AZT |
17 |
15 |
09 |
04 |
|
4. |
62 |
F |
8 months |
7 months |
Pred. RTX AZT |
24 |
20 |
11 |
07 |
|
5. |
43 |
M |
5 months |
4 months |
Pred. RTX MMF |
23 |
19 |
18 |
13 |
|
6. |
32 |
F |
18 months |
7 months |
Pulse MP RTX AZT |
16 |
16 |
10 |
06 |
|
7. |
46 |
F |
6 months |
8 months |
Pulse Dexa Pred. AZT |
22 |
17 |
09 |
04 |
|
8. |
26 |
F |
7 months |
7 months |
Pulse MP RTX Pred. |
15 |
14 |
10 |
03 |
|
9. |
41 |
F |
24 months |
4 months |
Pulse MP RTX Pred. AZT |
18 |
15 |
12 |
07 |
|
10. |
35 |
M |
8 months |
5 months |
Pulse Dexa Pred. AZT |
20 |
16 |
14 |
08 |
|
11. |
42 |
F |
9 months |
6 months |
Pulse MP Pred. MMF |
18 |
15 |
13 |
06 |
|
12. |
38 |
F |
15 months |
7 months |
Pred. RTX AZT |
19 |
14 |
15 |
09 |
|
13. |
47 |
F |
10 months |
3 months |
Pulse MP RTX Pred. AZT |
17 |
12 |
18 |
11 |
|
14. |
45 |
F |
5 months |
4 months |
Pred. AZT |
13 |
16 |
11 |
05 |
|
15. |
40 |
F |
8 months |
8 months |
Oral MP Pred. RTX AZT |
23 |
18 |
15 |
09 |
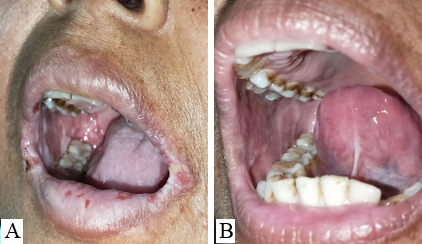
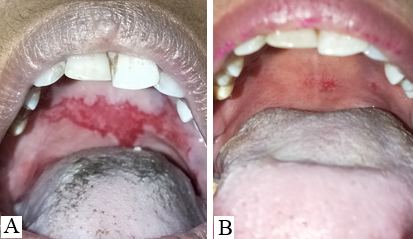
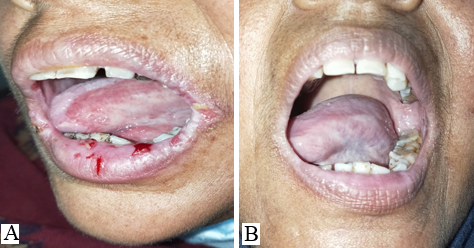
Discussion
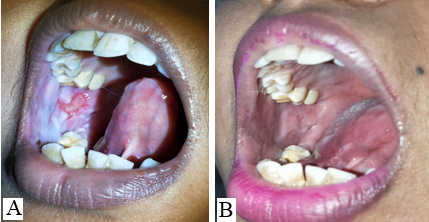
The mucosal erosions of Pemphigus Vulgaris are often the most difficult sites to treat for dermatologists as compared to the cutaneous lesions.[7], [8] The oral lesions severely hamper the day-to-day activity of the patients like brushing, eating, swallowing, and talking which affects the dietary intake of the patients. Improper nutrition of the patients delays the recovery and adds on to the disease burden. [9] Oral, I/L and topical corticosteroids have to be continued for a longer duration even after the skin lesions have subsided. The use of ILS has been found to be effective in treating recalcitrant oral erosions, [7] nonetheless, few patients do not respond to it satisfactorily. In this pilot study, we aimed to evaluate the efficacy of I/L autologous PRP in recalcitrant oral lesions of PV. It was observed that POLIS (Pemphigus oral lesion intensity score) significantly reduced after 3 sessions of I/L PRP therapy. Poor quality of life has a significant impact on treatment outcome of pemphigus. [10] Also, the overall quality of life of cases improved significantly.
PRP contains a milieu of growth factors & cytokines that promotes the healing process and has been used in treatment of chronic, non-healing ulcers being an easy, day care and cost effective procedure.[11] PRP has also been used in various oral surgeries & regenerative endodontic procedures due to its effect of wound healing and maintaining pulp vitality.[12]
I/L Rituximab and I/L methotrexate have been tried for recalcitrant oral lesions with variable results. Vinay et al[13] reported 3 OPV patients who had inadequate response to ILS but responded to I/L Rituximab injection. Another study conducted by Hrin, Mathhew et al on cases of mucous membrane pemphigoid showed promising results with I/L methotrexate.[14]
Taking into account that POLIS measures all the aspects of oral lesions of PV like sites, extent, duration, pain and difficulty in day to day activities; gradual reduction of POLIS in the study participants can be attributed to wound healing properties of PRP where it has shown to accelerate soft tissue healing. Also, it prevents the patients from side effects of I/L and topical corticosteroids like soft tissue atrophy, [15] secondary bacterial and fungal infections like-candidiasis, granuloma formation & gingival neovascularization.[16]
In conclusion, in this preliminary study, there appears to be significant improvement in recalcitrant ulcers of oral PV with I/L PRP therapy and no major side effects apart from pain and discomfort during injection was seen in the study participants. However, because of small sample size, further large-scale studies are needed to confirm our findings and explore the potential of PRP therapy as an adjunct to management of mucosal lesions of PV. We suggest that I/L autologous PRP must be used for treatment of mucosal erosions of PV cases who develop side effects to I/L and topical corticosteroids and when I/L and oral corticosteroids are contraindicated.
Source of Funding
None.
Conflict of Interest
None.
References
- Shamim T, Varghese VZ, Shameena PM, Suddha S. Pemphigus vulgaris in oral cavity: clinical analysis of 71 cases. Med Oral Patol Oral Cir Bucal. 2008;13(10):622-6. [Google Scholar]
- El-Komy M, Hassan A, Raheem H, Doss S, El-Kaliouby M, Saleh N. Platelet-rich plasma for resistant oral erosions of pemphigus vulgaris: A pilot study. Wound Repair Regen. 2015;23(6):953-5. [Google Scholar]
- Saha S, Patra A, Gowda S, Mondal N, Rahaman S, Ahmed S. Effectiveness and safety of autologous platelet-rich plasma therapy with total contact casting versus total contact casting alone in treatment of trophic ulcer in leprosy: An observer-blind, randomized controlled trial. Indian J Dermatol Venereol Leprol. 2020;86(3):262-71. [Google Scholar]
- Xu P, Wu Y, Zhou L, Yang Z, Zhang X, Hu X. Platelet-rich plasma accelerates skin wound healing by promoting re-epithelialization. Burns Trauma. 2020;8. [Google Scholar] [Crossref]
- Sindhuja T, De D, Handa S, Goel S, Mahajan R, Kishore K. Pemphigus Oral Lesions Intensity Score (POLIS): A Novel Scoring System for Assessment of Severity of Oral Lesions in Pemphigus Vulgaris. Front Med (Lausanne). 2020;7. [Google Scholar] [Crossref]
- Gregoriou S, Efthymiou O, Stefanaki C, Rigopoulos D. Management of pemphigus vulgaris: challenges and solutions. Clin Cosmet Investig Dermatol. 2015;8:521-7. [Google Scholar] [Crossref]
- Mignogna M, Fortuna G, Leuci S, Adamo D, Orabona GD, Ruoppo E. Adjuvant triamcinolone acetonide injections in oro-pharyngeal pemphigus vulgaris. J Eur Acad Dermatol Venereol. 2010;24(10):1157-65. [Google Scholar]
- Gharote H, Nair P, Kasetty S, Thomas S, Kulkarni A. Pemphigus vulgaris-a report of three cases. BMJ Case Rep. 2012. [Google Scholar] [Crossref]
- Kanwar A, De D. Pemphigus in India. Indian J Dermatol Venereol Leprol. 2011;77(4):439-49. [Google Scholar]
- Baican A, Chiorean R, Leucuta D, Baican C, Danescu S, Ciuce D. Prediction of survival for patients with pemphigus vulgaris and pemphigus foliaceus: a retrospective cohort study. Orphanet J Rare Dis. 2015;10. [Google Scholar] [Crossref]
- Dougherty E. An evidence-based model comparing the cost-effectiveness of platelet-rich plasma gel to alternative therapies for patients with nonhealing diabetic foot ulcers. Adv Skin Wound Care. 2008;21(12):568-75. [Google Scholar]
- Meschi N, Castro AB, Vandamme K, Quirynen M, Lambrechts P. The impact of autologous platelet concentrates on endodontic healing: a systematic review. Platelets. 2016;27(7):613-33. [Google Scholar]
- Vinay K, Kanwar AJ, Mittal A, Dogra S, Minz R, Hashimoto T. Intralesional rituximab in the treatment of refractory oral pemphigus vulgaris. JAMA Dermatol. 2015;151(8):878-82. [Google Scholar]
- Matthew L, JW, Bowers N, Ahn C, Strowd L. Methotrexate for oral mucous membrane (cicatricial) pemphigoid: experience at an academic dermatology outpatient clinic-Hrin. J Am Acad Dermatol. 2022;87(6):1431-3. [Google Scholar]
- Reddy P, Zelicof S, Ruotolo C, Holder J. Interdigital neuroma. Local cutaneous changes after corticosteroid injection. Clin Orthop Relat Res. 1995. [Google Scholar]
- Fortuna G, Mignogna MD. Clinical guidelines for the use of adjuvant triamcinolone acetonide injections in oro-pharyngeal pemphigus vulgaris: the oral medicine point of view. J Oral Pathol Med. 2011;40(4):359-60. [Google Scholar]
How to Cite This Article
Vancouver
Hussain MM, Tiwary PK, Singh A. Role of intralesional platelet-rich plasma (PRP) therapy in the treatment of recalcitrant oral ulcers of pemphigus vulgaris [Internet]. IP Indian J Clin Exp Dermatol. 2024 [cited 2025 Oct 05];10(4):442-447. Available from: https://doi.org/10.18231/j.ijced.2024.077
APA
Hussain, M. M., Tiwary, P. K., Singh, A. (2024). Role of intralesional platelet-rich plasma (PRP) therapy in the treatment of recalcitrant oral ulcers of pemphigus vulgaris. IP Indian J Clin Exp Dermatol, 10(4), 442-447. https://doi.org/10.18231/j.ijced.2024.077
MLA
Hussain, Md. Mobarak, Tiwary, Pankaj Kumar, Singh, Anupama. "Role of intralesional platelet-rich plasma (PRP) therapy in the treatment of recalcitrant oral ulcers of pemphigus vulgaris." IP Indian J Clin Exp Dermatol, vol. 10, no. 4, 2024, pp. 442-447. https://doi.org/10.18231/j.ijced.2024.077
Chicago
Hussain, M. M., Tiwary, P. K., Singh, A.. "Role of intralesional platelet-rich plasma (PRP) therapy in the treatment of recalcitrant oral ulcers of pemphigus vulgaris." IP Indian J Clin Exp Dermatol 10, no. 4 (2024): 442-447. https://doi.org/10.18231/j.ijced.2024.077
