- Visibility 282 Views
- Downloads 10 Downloads
- DOI 10.18231/j.ijced.2019.042
-
CrossMark
- Citation
Clinico -laboratory and trichoscopic evaluation of various patterns of female baldness – A prospective case control study
- Author Details:
-
Sappa Ramatulasi
-
Manjari Annapurna Malladi *
-
Ruttala Sri Satya
-
Kashetty Srujan Kumar
-
Anand Acharya
Introduction
Hair loss can potentially result in low self- esteem and poor body imge especially in women. Female pattern hair loss (FPHL) is a form of non scarring diffuse hair loss and hair thinning in a pattern fashion and can affect women as early as in their teen years.[1] Genetic predisposition & hormonal abnormalities like hyperandrogenism, hair cycle defects and follicular miniaturization have been implicated as etiopathogenetic factors for androgenic alopecia. There is a difference in the pathogenesis of patterned baldness in men and women. The role of hyperandrogenemia is not very clear in FPHL.[2] An association between iron deficiency and FPHL is also debated. Trichoscopy as a tool for evaluating hair loss disorders had gained momentum for the past one decade. However, studies investigating the features of patterned hair loss in females are few. This study was aimed to analyse the clinico- laboratory findings in women with pattern hair loss and to correlate the trichoscopic parameters in the three form s of female pattern hair loss namely Ludwig, Olsen, Hamilton & Norwood.
Materials and methods
Cases
Seventy female (70) patients of age between 18 to 48 years with history of hair loss over the scalp with the clinical features of pattern hair loss like thinning of hair over the crown, temporal area, recession of hair line, widening of central partition were included in the study after taking informed consent. The study has been approved by the institutional ethics committee.
Exclusion Criteria
Pregnant women, lactating mothers, postmenopausal or hysterectomised patients were excluded.
Patients with known endocrine disorders or those who are on hormonal therapies like oral contraceptive pills were excluded.
Patients on oral or topical treatments for hair loss were not included in the study.
Patients on drugs causing hair loss such as steroids, immunosuppressives, retinoids, anti cancer drugs were also excluded.
Patients with other types of cicatricial, non cicatricial alopecia and any hair shaft disorders were excluded
Controls
Thirty (30) female patients of age between 18 to 48 years with no clinical signs of hair loss or hyperandrogenism were included in the control group after taking informed consent.
History was obtained from each patient using a standard proforma which included age, marital status, parity, age of onset of hair loss, duration, menstrual irregularities.
Cutaneous examination was done to look for signs of hyperandrogenism like acne, hirsutism, acanthosis nigricans, striae. Scalp and hair were examined to rule out any signs of inflammation or scarring or any hair shaft abnormalities. The extent and pattern of hair loss was assessed by the severity of hair loss on the crown, recession of frontal hair line, involvement of fronto- temporal area and the patient s were then classified i n to Ludwig,[3] Olsen,[4] Hamilton & Norwood[5] types.
Trichoscopy was done using Dermaindia Wifi 2.0 dermoscope. Frontal, temporal, vertex, occipital areas were observed and images captured with the help of iphone 6 for analysis. Image capturing was performed by a single person to avoid variation. Morning blood sample was collected for hormonal and biochemical analysis on 3rd to 5th day of the menstrual cycle from the patients with regular cycles or on any other day from the patient who haven’t menstruated in the past 2months.
Total testosterone, dehydroepiandrosterone acetate(DHEAS), LH / FSH, prolactin, T3, T4, TSH, serum ferritin were done.
Ultrasound pelvis was done between 3rd and 5th day of menstrual cycle. A diagnosis of PCOS was done based on modified National I nstitute of Health (NIH) criteria.[6]
The data was tabulated in excel sheets and analysed using SPSS 16.0 version. The parametric data was analysed by unpaired ‘t’ test and non parametric data by Chi Square test. P value <0.05 was considered statistically significant. The results were expressed in mean, standard deviation.
Results
Out of the 70 patients, majority 42(60 % ) were in 29 t o 38 year age group followed by 15(21.43%) in 18 to 28 year age group and 13(18.57%) in 39 to 48year age group ([Table 1] ). T he mean age at presentation was 33 years (sd ± 4.5 ). The mean age of control group was 31.7 years (sd ± 3.2).
The mean age of onset of hair fall was 26.6(sd ± 2.4) years and the mean duration of hair loss was 4.8 (sd ± 1.8) years. In more than half of the patients,(53%) the duration of hair fall was 2 to 4 years where as it was less than 2 years in 30% of patients and the rest 17% of patients had hair loss for more than 4 years.
| Age in years | Cases (n= 70) | Controls (n=30) |
| 18-28 | 15 (31.43 % ) | 6 (20%) |
| 29-38 | 42 (60%) | 16 (53.3%) |
| 39-48 | 13 (18.57 %) | 8 (26.66 %) |
Family history of pattern hair loss was positive in 56% of patients. After detailed examination of scalp, t he patients were classified in to the three patterns of FPHL ([Table 2] ) .
| Variables | Number | Percentage | |
| Pattern of hairloss | Ludwing | 48/70 | 68.5% |
| Olseci | 18/70 | 25.8% | |
| Hamilton and Narwood | 4/70 | 5.7% | |
| Cutaneous findings | Acne | 20/70 | 28.5% |
| Acanthosis nigracans | 26/70 | 37% | |
| Hirsutism | 28/70 | 40% |
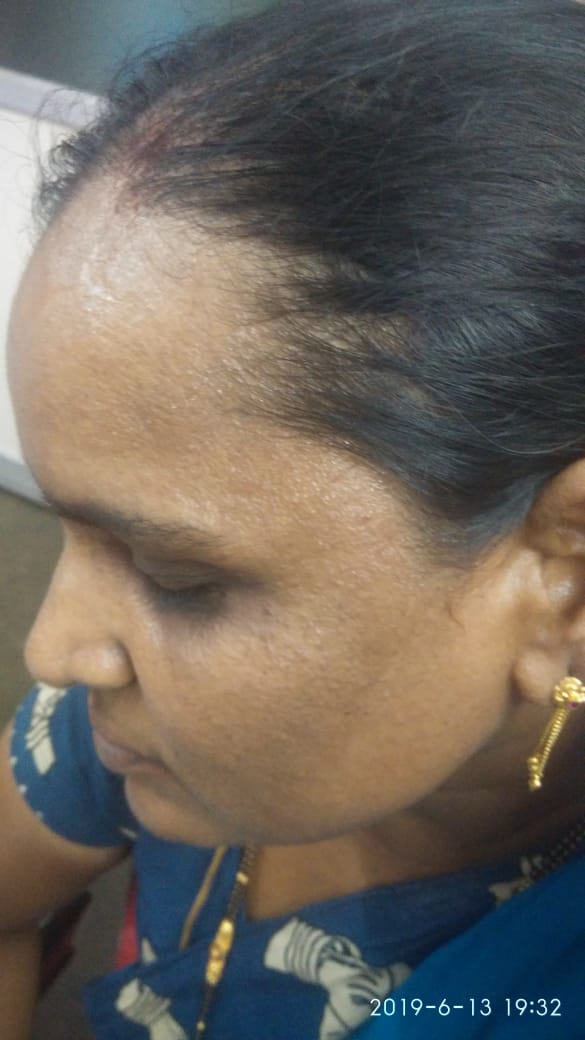
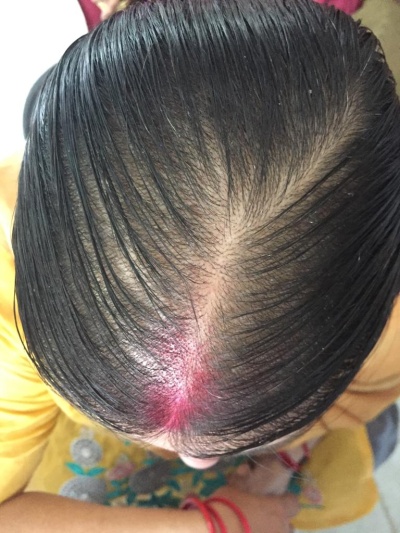
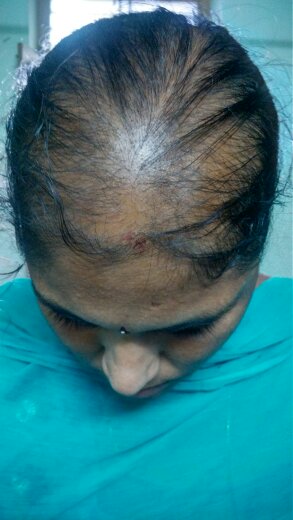
PCOS was diagnosed by modified NIH criteria in 19 patients (27.14 %) of the total cases.
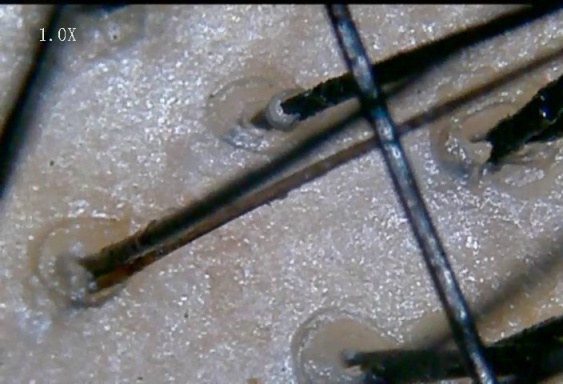
The parameters observed on trichoscopy were ([Figure 1],[Figure 2],[Figure 3],[Figure 4],[Figure 5] )
Hair diameter diversity (HDD): corresponds to vellus hair transformation
Brown peripilar sign (BPPS): subtle brown halo around the hair shaft
White peripilar sign (WPPS): larger in size as white halo at the follicular ostium
Yellow dots (YD) are are follicular infundibula with keratotic material and/or sebum
Focal atrichia: They are areas of total hair loss on scalp, usually in a size of a pencil eraser
Scalp honeycomb pigmentation (SHCP ): which correspon ds to melanotic rete ridges
Single follicular unit(SFU)




| Total no. of cases (n=70) | Ludwig (n=48) | Olsen (n=18) | Hamilton &Norwood (n=4) | ‘P’ value |
| Yellow dots (40) | 27 (56.21) | 10 (55%) | 3 (75%) | 0.82(>0.05) |
| BPPS (33) | 23 (48%) | 8 (44.4) | 2(50%) | 0.96(>0.05) |
| WPPS (31) | 20 (41.6%) | 9(50%) | 2(50%) | o.51(>0.05) |
| HDD (63) | 43 (90%) | 16 (89.5%) | 4 (100%) | 0.79(>0.05) |
| SFU (56) | 37(77%) | 16 (88%) | 3 (75%) | 0.56(>0.05) |
| WD (29) | 19 (39.5%) | 9 (50%) | 1 (25%) | 0.64(>0.05) |
| Scalp pigmentation(42) | 29 (63%) | 11 (66%) | 2 (50%) | 0.91(>0.05) |
| Focal atrichia (11) | 7 (14.5) | 3 (16.6%) | 1 (25%) | 0.97(>0.05) |
| HORMONE | Units | Cases Mean±sd | Controls Mean±sd | P VALUE |
| Total testosterone | ng/dl | 52.7 ±19.75 | 35.2 ±12.36 | <0.05 # |
| DHEAS | mic.gm/dl | 179 ± 61.27 | 154.56 ±52.14 | <0.05 # |
| T3 | ng/dl | 121.52 ±48.07 | 123.56 ± 36.04 | >0.05 |
| T4 | mic.gm/dl | 7.9 ±2.72 | 7.72±1.98 | >0.05 |
| TSH | Micro IU/ml | 6.87±1 | 3.17±1.97 | <0.05 # |
| LH/FSH ratio | 0.8±0.32 | 0.75±0.58 | >0.05 | |
| PROLACTIN | ng/lit | 16.95 ±6.22 | 14.53 ± 7.26 | >0.05 |
| S.FERRITIN | microgm/lit | 43.74 ±25.77 | 51.03 ± 29.2 | >0.05 |
Discussion
In this study, majority of the patients(60%) were in the age group of 29 -38 years similar to the study done in Tandon S et al[2] where in more than half of the patients were in 28-37 year age group. Similar observations were made by Lee et al[7] in their study of 445 patients out of which 252(56.68%) were in the third decade of their life. The mean age of presentation in the present study was 33 years (sd ±4.5). It was 37 years in a recent study by Ummita A et al[8] while it was 35.8 years in Hu et al[9] study done on Chinese population.
The mean age of onset of hair fall was 26.6(sd ±2.4) y ears in our study which is nearer to the study b y Tandon S et al[2] (26.1 years). In a study conducted by Shilpashree et al[10] mean age of onset was 28.4±8.2 years which is very similar to our study. Norwood[11] reported that Caucasian women had FPHL which begins in late 20s which is in accordance with this study. The mean age of onset was little high (29.8 years)in a study by Zhang et al[1] on Chinese women and it was little less (23 years) in the study done by Kasick et al[12]
The mean duration of hair fall was 4.8 years (sd±1.8 ) in the present study which is well in agreement with the studies of Zhang et al [1] and Tandon S et al [2] where the mean duration was 5.1 years and 4.49 years respectively.
Family history of hair loss was noticed in 56% of our patients which is similar to the study conducted by Shilpashree et al[10] at 51%. It varied from 19.2 to 32.4% in various Chinese studies.[11] [13] The incidence of family history of baldness was 45.2% in the study by Paik et al[14] on Korean women and 45% in the study by Zhang et al[1] on Chinese women which are in near agreement with our study. In the study by Tee Wei Siah et al[15] 85% of patients had family history of baldness.
The cutaneous findings of hyperandrogenism observed in assosciation with FPHL in our patients were Hirsutism (40%), Acanthosis nigracans (37%) and acne (28.5 %)
Most of the previous studies reported hirsutism in 12-22% of patients.[16],[17],[18] where as Tandon S et al [2] reported hirsutism in 66.6% of their patients.
Cela et al [16] reported acne in 38 of 80 patients (43%) and Moltz[17] and Tandon S et al[2] observed acne in 41.6% and 36.6% of their patients respectively.
43.3% of patients with FPHL had acanthosis nigracans in the study by Tandon S et al [2] which is little higher than that observed in our study (37%).
P COS was diagnosed based on modified NIH criteria in 19 of the total 70 patients (27.14%). Cela et al[16] at the reproductive endocrinology service in London reported a higher prevalence of PCOS (67)% while Quinn et al[19] and [2] Tandon S et al[2] reported 22% and 26.6% respectively which is similar to the present study. This difference in the prevalence could be due to different population groups studied, difference in sample size and screening methods used in diagnosis.
Various studies have evaluated the role of hyperandrogenemia in FPHL but the results were discordant. Molly Quinn et al[19] did not find a difference in overall biochemical hyperandrogenemia between subjects with and without AGA. Similarly, Ozdemir et al[20] concluded that AGA was not a marker for hyperandrogenemia. In contrast, Cela et al[16] reported a higher total testosterone, androstenedione and free testosterone index in 89 women with AGA compared with 73 control women.
In the present study, the difference between the mean values of total testosterone, DHEAS and TSH of cases were statistically significant when compared with that of controls (Z-test p<0.05) which is similar to the observations made by Tandon et al.[2] Kasick et al[12] also reported a significant increase in DHEAS levels in cases compared to controls. However, total serum testosterone levels were normal in all their subjects whereas 19% of our patients had serum total testosterone above the normal range.
DHEAS, apart from conversion to potent androgens, has a direct action on hair follicle as inhibitor of G6PD (glucose 6 phosphate dehydrogenase) there by inhibiting nucleic acid synthesis. This suggests that the se low potent adrenal androgens are sufficient to cause FPHL with or without other findings of hyperandrogenism. However, Montalto et al[21] and Rushton et al[18] did not find a significant increase in the plasma levels of DHEAS in their studies.
TSH and prolactin can interact with androgen metabolism at various levels. Futterweit et al[22] reported that two patients had hyperprolactinemia out of the 109 female patients with diffuse alopecia. Schimdt et al[23] had studied hypoth yroidism and hyperprolactinemia as a possible cause of androgenetic alopecia in females and found that 23% of their patients had raised TSH which is in accordance with our study where 28.5% patients had raised TSH levels. One third of (2 9%) their patients had raised prolactin. In contrast, all our patients had normal prolactin levels.
The relationship between serum ferritin and hair loss has been investigated in a number of studies however with relatively discrepant findings. Sinclair[24] in his study found no direct relationship between low serum ferritin and hairloss. Rasheed et al[25] found that serum ferritin levels in TE and FPHL cases were significantly lower than in controls. There is a wide range of serum ferritin level (6-160 mic.gm/l ). However, as per the literature a cut off of 41 mic.gm/l yields specificity of 98% and sensitivity of 98%. When this cut off level was taken, more than half (52%) of our patients fall under this group which is comparable to Olsen et al[26] (58.8%) and Rushton et al[18] studies( 65%) indicating an association between Iron deficiency and FPHL. In our study, t he mean serum ferritin level of patients were less than that of controls but this was not statistically significant.
The patients were classified in to three patterns of hair loss ([Table 2] ) and the trichoscopic findings in each pattern were as shown in [Table 4] .
Hair shaft diameter diversity (HDD) was established by Rakowska et al[27] as one of the major criteria of FPHL. It was observed in 93% of our patients similar to the study done by Galliker and Treub et al[28] where HDD was seen in 72% of early and 100% of advanced female androgenetic alopecia patients.
Brown peripilar sign ( BPPS) was seen in 47% of our patients similar to Ummiti A et al[8] 40% and Hu et al[9] (44.5%) studies. It was 31.7% in a Chinese study by Zhang et al.[1] In contrast, Deloche et al[29] reported it to be 86% in caucasian women with FPHL and Inuis et al30 detected this finding in 20%.
White peripilar sign ( WPPS) was noticed in 44.2% of patients in the present study. Its incidence varied from 15 to 68% in various studies [1],[8],[9]
Yellow dots(YD) were seen in 40 out of 70 pts (57%) in our study. Contrastingly its incidence was high (88%) in Ummiti A et al[8] and very low in Zhang et al[1] study(1.67%). Hu et al[9] observed this finding in 24% of FAGA patients. This disparity of findings can be due to different skin phenotypes with variation in sebaceous gland activity as well as degree of pigmentation of scalp as suggested by Ummiti A et al.[8]
Focal atrichia referred to as pencil erased focal loss of hair was found in 18.6% in our study nearer to that observed by Ummiti A et al[5] (24%) whereas Hu et al[9] & Zhang et al[1] reported its incidence in more than half of their patients. Zhang et al[1] correlated this finding with advancing stage o f androgenic alopecia.
Scalp honey comb pigmentation was observed in 60% patients similar to Zhang et al[1] (61.7%) study where as in a chinese study by Hu et al[9] it was seen in 30.5% and in Ummiti A et al[8] study it was seen in 80% of patients.
In addition to these, we observed white dots (representing empty follicles) in 41.4% of patients and single follicular units in 80% of patients. To the best of our knowledge, there are no previous studies comparing the trichoscopic findings in various forms of female baldness. As per our study, there is no stastically significant difference in the incidence of parameters of trichoscopy among the three patterns of hair loss.
Funding
None.
Conflict of Interest
None.
References
- X Zhang, S Caulloo, Y Zhao, B Zhang, Z Cai, J Yang. Female pattern hair loss: clinico-laboratory findings and trichoscopy depending on disease severity. Int J Trichology 2012. [Google Scholar]
- S Tandon, P Arora, R K Gautam, M Bhardwaj, U Garga, N Sharma. . Correlation between clinical features ,biochemical parameters, and histopathological findings in women . [Google Scholar]
- E Ludwig. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol 1977. [Google Scholar]
- E A Olsen. Androgenetic alopecia. Disorders of hair growth: diagnosis and treatment 1994. [Google Scholar]
- O T Norwood. Male pattern baldness:classification and incidence. South Med J 1975. [Google Scholar]
- R Azziz. Diagnostic criteria for polycystic ovary syndrome: a reappraisal. FertilSteril 2005. [Google Scholar]
- W S Lee, B I Ro, S P Hong, H Bak, W Y Sim, D W Kim. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am AcadDermatol 2007. [Google Scholar]
- A Ummiti, Priya Ps, P L Chandravathi, C S Kumar. Correlation of trichoscopic findings in androgenic alopecia and the disease severity. Int J Trichol 2019. [Google Scholar]
- R Hu, F Xu, Y Han, Y Sheng, S Qi, Y Miao. Trichoscopic findings of androgenetic alopecia and their association with disease severity. J Dermatol 2015. [Google Scholar]
- P 10 Shilpashree, - Ravikiran. An epidemiological study of female pattern hair loss at a referral centre in South India Indian. Journal of Clinical and Experimental Dermatology 2016. [Google Scholar]
- . 11 Norwood OT. Incidence of female androgenetic alopecia (female pattern alopecia). DermatolSurg 2001. [Google Scholar]
- J M Kasick, W F Bergfeld, W D Steck, M K Gupta. Adrenal androgenic female-pattern alopecia: sex hormones and the balding woman. Cleve Clin Q 1983. [Google Scholar]
- M E Gonzalez, J Cantatore-Francis, S J Orlow. Androgenetic alopecia in the paediatric population: A retrospective review of 57 patients. Br J Dermatol 2010. [Google Scholar]
- J H Paik, J B Yoon, W Y Sim, B S Kim, N I Kim. The prevalence and types of androgenetic alopecia in Korean men and women. Br J Dermatol 2001. [Google Scholar]
- 15 Tee Wei Siah ., Llorenia Muir-Green .. Jerry Shapiro Female Pattern Hair Loss: A Retrospective Study in a Tertiary Referral Center. Int J Trichology 2016. [Google Scholar]
- E Cela, C Robertson, K Rush, E Kousta, D M White, Wilson ., H .. Prevalence of polycystic ovaries in women with androgenic alopecia. Eur J Endocrinol 2003. [Google Scholar]
- . 17 Moltz L. [Hormonal diagnosis in so-called androgenetic alopecia in the female. GeburtshilfeFrauenheilkd 1988. [Google Scholar]
- D H Rushton, I D Ramsay, K C James, M J Norris, J J Gilkes. Biochemical and trichological characterization of diffuse alopecia in women. Br J Dermatol 1990. [Google Scholar]
- M Quinn, K Shinkai, L Pasch, L Kuzmich, M Cedars, H Huddleston. Prevalence of androgenic alopecia in patients with polycystic ovary syndrome and characterization of associated clinical and biochemical features. FertilSteril 2014. [Google Scholar]
- S Ozdemir, M Gorkemli, H Kiyici, A Bodur, S .. Specific dermatologic features of the polycystic ovary syndrome and its association with biochemical markers of the metabolic syndrome and hyperandrogenism. ActaObstetGynecol Scand 2010. [Google Scholar]
- J Montalto, C B Whorwood, J W Funder, A B Yong, A Callan, H E Davies. Plasma C19 steroid sulphate levels and indices of androgen bioavailability in female pattern androgenic alopecia. ClinEndocrinol (Oxf) 1990. [Google Scholar]
- W Futterweit, A Dunaif, H C Yeh, P Kingsley. The prevalence of hyperandrogenism in 109 consecutive female patients with diffuse alopecia. J Am AcadDermatol 1988. [Google Scholar]
- J B Schmidt, B Schurz, J Huber, J Spona. Hypothyroidism and hyperprolactinemia as a possible cause of androgenetic alopecia in the female. Z Hautkr 1989. [Google Scholar]
- R Sinclair. There is no clear association between low serum ferritin and chronic diffuse telogen hair loss. Br J Dermatol 2002. [Google Scholar]
- H 25 Rasheed, D Mahgoub, R Hegazy, M El-Komy, Abdel Hay, R Hamid, M A .. Serum ferritin and Vitamin D in female hair loss: Do they play a role?. Skin PharmacolPhysiol 2013. [Google Scholar]
- E A Olsen, K B Reed, P B Cacchio, L Caudill. Iron deficiency in female pattern hair loss, chronic telogen effluvium, and control groups. J Am AcadDermatol 2010. [Google Scholar]
- A 27 Rakowska, M Slowinska, E Kowalska-Oledzka, M Olszewska, L Rudnicka. Dermoscopy in female androgenic alopecia: Method standardization and diagnostic criteria. Int J Trichology 2009. [Google Scholar]
- N A Galliker, R M Treb. Value of trichoscopy versus trichogram for diagnosis of female androgenetic alopecia. Int J Trichology 2012. [Google Scholar]
- C Deloche, O De Lacharrire, C Misciali, B M Piraccini, C Vincenzi, P Bastien. Histological features of peripilar signs associated with androgenetic alopecia. Arch Dermatol Res 2004. [Google Scholar]
How to Cite This Article
Vancouver
Ramatulasi S, Malladi MA, Satya RS, Kumar KS, Acharya A. Clinico -laboratory and trichoscopic evaluation of various patterns of female baldness – A prospective case control study [Internet]. IP Indian J Clin Exp Dermatol. 2025 [cited 2025 Sep 04];5(3):195-201. Available from: https://doi.org/10.18231/j.ijced.2019.042
APA
Ramatulasi, S., Malladi, M. A., Satya, R. S., Kumar, K. S., Acharya, A. (2025). Clinico -laboratory and trichoscopic evaluation of various patterns of female baldness – A prospective case control study. IP Indian J Clin Exp Dermatol, 5(3), 195-201. https://doi.org/10.18231/j.ijced.2019.042
MLA
Ramatulasi, Sappa, Malladi, Manjari Annapurna, Satya, Ruttala Sri, Kumar, Kashetty Srujan, Acharya, Anand. "Clinico -laboratory and trichoscopic evaluation of various patterns of female baldness – A prospective case control study." IP Indian J Clin Exp Dermatol, vol. 5, no. 3, 2025, pp. 195-201. https://doi.org/10.18231/j.ijced.2019.042
Chicago
Ramatulasi, S., Malladi, M. A., Satya, R. S., Kumar, K. S., Acharya, A.. "Clinico -laboratory and trichoscopic evaluation of various patterns of female baldness – A prospective case control study." IP Indian J Clin Exp Dermatol 5, no. 3 (2025): 195-201. https://doi.org/10.18231/j.ijced.2019.042
